Washington University’s Program in Psychedelic Research
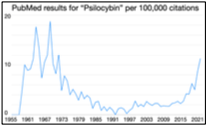
Since 2005, the percent of adults in the US with depression and anxiety disorders has risen steadily, a trend that accelerated in the COVID-19 pandemic. These disorders are now the leading cause of disability worldwide, resulting in high costs to individuals, families and society. While current treatments help many people, they have many drawbacks including slow onset of effect, significant side-effects, and lack of efficacy in at least 30% of depressed patients. Thus, there is a major need for novel and effective therapeutic agents. A class of drugs called indole-amine psychedelics has gained a great deal of recent attention as potentially curative treatments, with the FDA granting breakthrough therapy designation to two large clinical trials of psilocybin for depression (Compass Pathways and Usona Institute).
The Program in Psychedelic Research (PiPeR) is a partnership between The Healthy Mind Lab, the Washington University (WU) Neuroimaging Labs, and Usona Institute. It leverages world-class expertise in clinical trial design, human neuroimaging and pre-clinical science to understand how psychedelic medicines change the human brain. Our research will lead to the next generation of safer and more effective treatments for psychiatric illness.
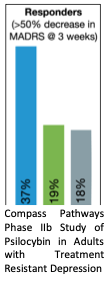
Psychedelics produce rapid, transformative, and durable antidepressant effects. After nearly half a century of stagnation, clinical interest in psychedelics was reignited in the late 2000’s after a study in healthy adults demonstrated beneficial changes in personality and mood following a single dose of psilocybin. Subsequent clinical trials have demonstrated immediate and sustained symptom relief in depression, anxiety, and substance use disorders following a dose of psilocybin with therapy. In the largest clinical trial to date (N=233), Compass Pathways recently reported a dramatic improvement 3 weeks after a single dose of psilocybin (25mg vs 1mg) for treatment resistant depression.
Depression is associated with decreased neuroplasticity. This is termed “the neurotrophic hypothesis.” Both animal and human research converge on the observation that stress and depression are linked to reduced volume and decreased density of neurons and synapses in key brain areas (prefrontal cortex and hippocampus). Antidepressants stimulates neurogenesis and neuroplasticity in these areas, reversing the changes caused by stress. This is a common observation across multiple classes of antidepressant drugs and seems to be both necessary and sufficient for recovery from depression.
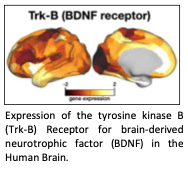
Psychedelics powerfully stimulate neuroplasticity and synaptogenesis. In paradigms comparing across antidepressants, psilocybin produces a larger magnitude and longer lasting neurotrophic effect of any other antidepressant drug. Yet, the impact of neuroplasticity at the macro scale is unknown. Large-scale brain networks are the missing link between neurotrophic activation, and the powerful antidepressant effects of psychedelics.
The Neuroimaging Labs at Washington University (WU) has been a world leader in brain mapping research for over 30 years. WU was the site of the first cyclotron developed for medical PET imaging research and the first PET scanners. WU Neuroimaging Labs developed resting state fcMRI as a tool for brain network mapping, led the Human Connectome Project, and most recently developed Precision Functional Mapping – a method for mapping brain networks in high resolution at the individual level. The traditional approach to brain mapping research has been to average brain connectivity across a study cohort. We now see that, because of the uniqueness of individual brains, this was a flawed approach. Imagine average across 20 individuals’ MRIs to assess heart function. Precision Functional Mapping, by map brain networks at the individual level, has observed effect sizes 500% larger than traditional approaches, leading to paradigm shift in brain imaging science.
Core Partnerships in the Program in Psychedelic Research
The Healthy Mind Lab, has led Precision Psychotherapeutics Research within the Department of Psychiatry for four decades. Our psychedelics research team (the Healthy Mind Lab), co-lead by Drs. Josh Siegel and Ginger Nicol, has decades of experience in clinical research and neuroimaging with antidepressants. This includes work with ketamine, with recent clinical trials focused on optimizing clinical response and understanding neurobiology.
Usona Institute is a not-for-profit medical research organization whose mission is to support clinical and preclinical research with psilocybin, In 2019, Usona Institute received a breakthrough therapy designation from the FDA for a clinical trial of psilocybin in adults with major depressive disorder (PSIL201). In 2020, the Healthy Mind Laboratory partnered with the Usona Institute to begin research elucidating the mechanisms by which psilocybin produces rapid antidepressant effects in humans. Our continued partnership with Usona has provided access to pharmaceutical-grade agents, training and assistance in psychedelic medication protocols and psychotherapy, safety monitoring, regulatory guidance. Our continued relationship provides tremendous benefits and serves as a model for academic-industry partnership.
Ginger Nicol, MD (Principal Investigator): Associate professor in psychiatry with extensive clinical trials experience.
Joshua Siegel MD/PhD (Project Leader). Neuroimager (h-index = 18). Joining WU Psychiatry faculty June 2022.
Dean Wong, MD/PhD (Co-Investigator). Professor of Radiology and world leader in brain PET imaging with 40 years’ experience, including imaging with psilocybin.
Charles Raison, MD (Co-Investigator). Endowed Professor of Psychiatry at the University of Wisconsin-Madison and Director of Clinical and Translational Research at Usona Institute.
Abraham Snyder, MD/PhD (Collaborator). World leader in fMRI (h-index = 132). Professor of Neurology.
Nico Dosenbach, MD/PhD (Collaborator). Professor of Neurology, pioneer of Precision Functional Mapping.
Eric Lenze, MD (Collaborator). Wallace & Lucille Renard Professor of Psychiatry and Director, Healthy Minds lab with 30 years of continuous NIH funded clinical research in psychiatry.
Adam Bauer, PhD & Jordan McCall, PhD (WU Collaborators): Junior Faculty, conducting research to understand the effects of 5-HT2A agonism and antagonism on neuronal and neurovascular signals in the mouse cerebral cortex.
The Future of Physician-Scientist Workforce Development

Unraveling the mysteries of how psychedelic medicines can so rapidly and profoundly change the lives of individuals suffering with neuropsychiatric conditions requires not only the right combination of expertise, technology, and infrastructure, but also experience working with regulatory agencies and institutional regulatory bodies to ensure safety and adherences to licensing and reporting requirements for conducting human subjects research. Our interdisciplinary team includes world-renowned experts in neuroscience, functional and molecular neuroimaging, neuropsychopharmacology and precision clinical trials, and uniquely spans the translational spectrum from preclinical animal studies to randomized clinical studies in young adults and adults. We are also the only clinical research group in the state of Missouri and one of less than 30 academic centers in the US with the capability to safely and legally conduct clinical research with schedule 1 medications like psilocybin. Finally, our team prominently highlights the work and career development of physician-scientists in all stages of training at WUSM, from undergraduate, graduate, and professional degree programs in neuroscience, neuroradiology, and psychiatry.
Current Research Studies
Psilocybin Precision Functional Mapping (Research Phase 1) In partnership with Usona, our group has established the safety and feasibility of a novel, intensive precision functional brain mapping protocol conducted before, during, and after psilocybin exposure (~20 MRI visits per subject) in healthy adults. By using advanced imaging and intensive scanning in the weeks following a psilocybin dose, this novel design will elucidate the time course of effects on individual brain networks. Data acquisition is ongoing and will answer critical questions about the acute and persisting effects of psychedelic medicines.
FDA IND: #154066, clinicaltrials.gov/ct2/show/NCT04501653
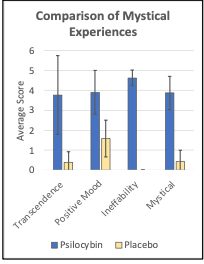
Progress: Three participants have completed the protocol, reporting dramatic mystical experiences (Figure 6), and generating highly reliable brain connectome data. This establishes the feasibility of conducting neuroimaging research with psilocybin at WUSM.
Psilocybin as a treatment for depression. Psilocybin has emerged as a promising new treatment for depression. The WUSM Program in Psychedelic Research is recruiting for a clinical trial evaluating the use of psilocybin in conjunction with psychological support as a treatment for major depression. If you are interested in learning more about the study or referring a patient, please contact davidabender@wustl.edu.
PET marker of synaptogenesis in a clinical trial of Psilocybin for depression (Research Phase 2). A recently discover radiotracer (11C-UCB-J) directly measures synaptic vesicle density, a marker of synapse growth/loss. Following demonstration of safety and utility in humans, studies have identified focal synaptic loss in epilepsy, dementia, schizophrenia, and depression. This discovery opens the door to directly measure the neurotrophic mechanism of psilocybin’s antidepressant activity. Critically, it also makes it possible to determine the relationship the perceptual (entheogenic, mystical) effects of psilocybin, and its neurotrophic effect. Partnered with a world leader in PET imaging (Dean Wong), we have successfully synthesized 11C-UCB-J at WU. Building from our preliminary study, and capitalizing on unique institutional resources, we have proposed the first-in-humans study to directly measure synaptogenesis as a mechanism of psilocybin’s antidepressant activity. NIL houses one of the only Biograph mMR scanners in the US, allowing for simultaneous acquisition of MRI and PET. Using the biograph mMR we can simultaneously map of brain network correlates of synaptogenesis.
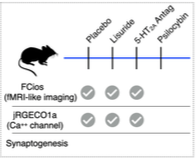
Animal Research to elucidate neurocircuitry of the serotonin 2A receptor (5-HT2A): Psychedelics’ perceptual effects occur via 5-HT2A agonism. However, recent research suggests that their hallucinogenic effects may be dissociable from neurotrophic effects. Elucidating the complex role of the 5-HT2A receptor in neural, neurovascular, and neurotrophic effects is critical in future development of psychedelic and related medicines. In this collaboration between Adam Bauer, PhD (Biomedical Engineering), Jordan McCall, PhD (Pharmacology) and Joshua Siegel, MD PhD (Psychiatry), we will differentiate the effects of 5-HT2A agonism by psychedelics from non-hallucinatory agonism with respect to neuronal, neurovascular, and neurotrophic effects. Using rodents allows us to define observed signals at level not accessible in ongoing human research.
Progress: We have collected a preliminary data in a cohort of mice including randomized exposure to either 1) saline, 2) Lisuride (non-hallucinatory 5-HT2A agonist), 3) M1 (5-HT2A antagonist), or 4) Lisuride + M1 followed by simultaneous neural and neurovascular imaging.
Usona Phase II/III Clinical Trial (PSIL201): We are currently in planning with Usona to be a site for their Phase II/III clinical trial of psilocybin 25mg for depression. This will provide tremendous synergy and added value with our ongoing human research and training efforts.
Funding: PiPeR will largely be fueled by support from NIH, Usona Institute, and Private Foundations. The core investigators have proven track records of successfully competing for NIH funding. However, federal support for psychedelics research remains limited. The National Institute of Mental Health has yet to fund any human psychedelics research. Further, the funding environment of NIH threatens high risk drug development research. Therefore, to develop psychedelics and psychedelic-related medicines into the next generation of treatments in psychiatry, philanthropic support is critical. By providing a unifying structure for these research studies, and academic-private sector partnership, PiPeR will provide a mechanism by which better treatments can move from concept to development.
