Our research aims to improve health outcomes by studying treatments that promote healthy minds and healthy bodies across your lifespan.
📰Healthy Mind Lab latest:
“Your brain on shrooms” – check us out in Nature and the New York Times!
Click the buttons below this post to check out both articles:
Washington University researchers test antidepressant for treating long COVID (Links to an external site)
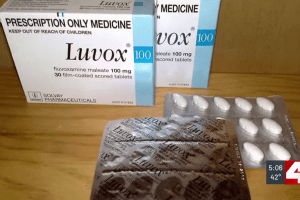
By Russell Kinsaul Published: Mar. 18, 2024 at 5:55 PM CDT Check out the Healthy Mind Lab’s current study on long COVID! Researchers at Washington University are leading the way to find a treatment for a debilitating condition that affects as many as 16 million Americans.
🔗Healthy Mind Lab Quick Links
📅Want to learn more about Psychiatry? Check out department lectures and presentations:
There are not currently any upcoming events scheduled. Please check back for new events to be added.
In the meantime, if you’d like to explore campus events, please visit Happenings at WashU.
🧠Can I volunteer for a Healthy Mind Lab study?
Healthy Mind Lab is always seeking volunteers for clinical trials. Click below to see if you may be a match!