Ipcs in TRD
Ipcs is a new device study for severe treatment resistant depression (TRD) - accepting applicants ages 21-80 years

An Early Feasibility Study Of Intracalvarial Prefrontal Cortical Stimulation (Ipcs) In Severe Treatment-Resistant Depression
What is involved in this study?
The purpose of this research study is to look the safety and efficacy of intracalvarial prefrontal cortical stimulation in treatment resistant depressed (TRD) persons.
What is intracalvarial prefrontal cortical stimulation?
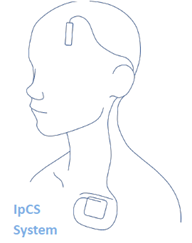
This is a minimally invasive treatment involving surgically placing an electrode device in your skull. Over time, electric pulses will be sent from the device to affect specific areas of the brain thought to be involved with depression.
What is TRD?
Treatment resistant depression (TRD) is depression that has not improved after trying 2 or more medications.
What will I need to do?
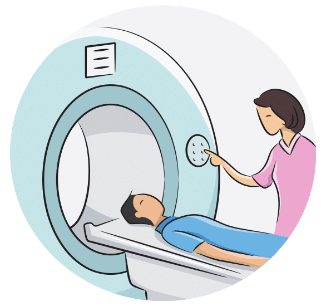
If you are eligible to continue onto the study, you will be asked to have a series of CT and MRI scans of your head down to identify a location to implant the device. You will also complete baseline assessments for data collection.
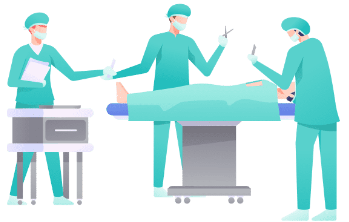
Once baseline assessments are completed, you will undergo surgery to have the device implanted within the thickness of your skull. Another device (a pulse generator) will be implanted in the right side of your chest, below your skin and above your chest muscle. A wire will also be passed just under the skin of your neck and a second wire will be placed under your scalp to connect the devices. Immediately after surgery a CT scan will be done to verify placement. Before you are discharged, the research team will adjust the device electronically to make sure they have the right stimulation setting for you.
Throughout the course of the study you will have about 25 study visits that will start weekly, then become bi-weekly, then become monthly. You will also have access to on-call phone numbers for Washington University Neurosurgery and Psychiatry on-call physician services for urgent study related issues throughout your participation.
Will it cost me anything to be in this study?
You will not have any costs for being in this research study.
How long will I be in this study?
Your total participation will last up to 18 months. Visits will look like the following:
Baseline Assessment and Image collection:
- 1 in-person assessment lasting about 4 hours
- 2 scanning sessions lasting about 2 hours each
- Baseline assessment and scanning sessions will occur over the course of 3 weeks
Surgical Procedure:
- The surgery itself is expected to last less than 3 hours
- You will have a CT scan done immediately after surgery
- You are expected to be discharged from the hospital within 24 hours of your surgery
Acute Phase:
- 4 weekly in-person assessments lasting about 2 hours

Continuation Phase:
- 7 weekly assessments lasting about 1-2 hours
- 4 bi-weekly assessments lasting about 1-2 hours
- 7 monthly assessments lasting about 1-2 hours
Am I eligible to participate in this study?
You may be eligible to participate in this study if:
- You have a diagnosis of chronic (greater than or equal to 2 years) or recurrent (multiple prior episodes) depression and are currently experiencing a major depressive episode as defined by DSM V criteria
- You have treatment resistant depression (TRD)
- You are between the ages of 21-80
If you are interested in participating, please contact:
Karen Flavin, RN, CRC | 314-747-6998 | karen.flavin@wustl.edu
